I always ask my outgoing sixth formers what activities they found the most memorable from their time at school. Almost without fail, ‘rainbow fizz’ is mentioned – an introductory acid–base practical that gives students a look at indicators and neutralisation. This got me thinking – if it’s so memorable, is it worth repeating for older students?
Kit
- 500 cm3 measuring cylinder
- Two 100 cm3 measuring cylinders
- Two 250 cm3 conical flasks
- Two magnetic stirrers
- Universal indicator solution (highly flammable, eye irritant, harmful by ingestion)
- Methyl orange indicator solution
- Phenolphthalein indicator solution, 0.1% w/v (highly flammable, eye irritant, harmful by ingestion)
- Sodium carbonate, c.30 g (irritant)
- Ethanoic acid, 200 cm3, 1 mol dm–3 (irritant)
- Hydrochloric acid, 100 cm3, 1 mol dm–3
- Ethanoic acid, c.10 cm3, 2 mol dm–3 (optional)
- Eye protection for the demonstrator
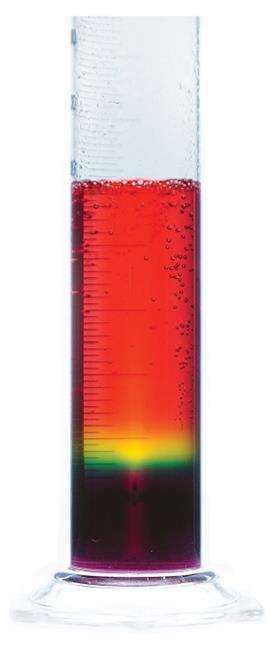
Giant rainbow fizz
To prepare, dissolve 20 g of sodium carbonate in 100 cm3 of water and add to the 500 cm3 measuring cylinder. Add 30 drops of universal indicator solution, which will turn purple. To each of the 100 cm3 measuring cylinders add 15 drops of universal indicator solution followed by 100 cm3 of 1 mol dm–3 ethanoic acid – these will turn red.
In front of the audience, point out the colours of the solutions – older students could predict the pH given the relevant pKa values. The sodium carbonate solution has a pH of 12.2 and the ethanoic acid a pH of 2.4. Add the first acid sample to the carbonate, pouring down the side of the cylinder – you can work reasonably quickly here as some mixing helps to develop a broader band of mid-range pH.
As the fizzing subsides, the second sample of acid can be added – this time slowly pouring down the side of the cylinder. Eventually, the large tube will contain a full spectrum of colours from purple at the bottom through to red at the top. Depending on how much mixing took place, you may need to add a little more ethanoic acid to see the red colour at the top.
Point out to the audience where the bubbles are appearing – they appear to form in the yellow zone (pH 6) and get significantly larger and faster as they rise through the increasingly acidic solution.
Demonstrating pH range
Prepare a second demo by dissolving 2.65 g of sodium carbonate in about 25 cm3 water in each of the 250 cm3 conical flasks placed on magnetic stirrers. Add 2 drops of phenolphthalein indicator solution to one and the same amount of methyl orange to the other. Measure out four aliquots of 1 mol dm–3 HCl – two of 21 cm3 and two of 23 cm3. Keep the reagent bottle of acid nearby.
When you’re in front of the class, set the stirring as fast as possible. Add the 21 cm3 sample of hydrochloric acid to each. The pink solution will turn colourless and the yellow solution will remain unchanged – virtually no CO2 will be produced.
On the addition of the next 23 cm3 of acid, the reaction will immediately fizz but the solutions will remain yellow and colourless. Wait a few moments for the effervescence to subside and then add a few more millilitres from the reagent bottle to get the final colour change to red.
Teaching goal
Indicators work as weak acids – the acid (HIn) and its conjugate base (In–) having different colours. When there are equal concentrations of the acid and conjugate base, the pH of the solution will match the pKa of the indicator (see the Henderson–Hasselbalch equation).
An accurate titration relies on the chemist choosing an indicator that changes colour at the equivalence point for the titration.
The pH curve for a sodium carbonate–acid titration has two equivalence points: one for the protonation of sodium carbonate, and one for the subsequent reaction of sodium bicarbonate with more acid.
The first equivalence is at pH 8.3. Phenolphthalein, with a pKa of 9.6, will have already decolourised by this point. This corresponds to the first addition of acid in the demonstration.
The second addition of acid carries the reaction through the first equivalence point to just before the second one and finishes around pH 5. In this stage of the reaction CO2 is released, but with no colour changes.
Adding more acid (approximately 10 cm3) to the flask containing methyl orange now takes it through the second equivalence point – showing how these two indicators would give vastly different values for the end point of a titration of sodium carbonate.
Safety and disposal
Wear eye protection. All liquids can be disposed of down the sink with plenty of water. Indicator solutions are likely made with industrial denatured alcohol (IDA). IDA is also an eye irritant, harmful by ingestion and causes damage on repeated or prolonged exposure to the optic nerve, CNS (and some other organs).
Downloads available for this article
Downloads
Rainbow fizz MS Powerpoint slides
PowerPoint, Size 6.6 mb













![5 ideas a5000682 copper oxide and zinc reaction spl 300tb[1]](https://d1ymz67w5raq8g.cloudfront.net/Pictures/100x67/6/1/0/113610_5ideas_a5000682copper_oxide_and_zinc_reactionspl_300tb1.jpg)




4 readers' comments