Nina Notman meets the teams exploring the potential of locking Earth’s excess carbon dioxide away for millions of years by turning it into rock
Locking Earth’s excess carbon dioxide away for millions of years by turning it into rock
Iceland is widely touted as a leader in green energy, generating 100% of its electricity from renewable sources. It might come as a surprise, then, to hear that this sparsely populated country has a huge carbon dioxide emissions problem. This is because although Iceland’s two electricity sources – hydropower and geothermal – are promoted as being clean, they still cause the emission of significant amounts of carbon dioxide and other gases.
A secondary issue is that Iceland produces a lot of electricity for industrial purposes, explains Eric Oelkers, professor of geochemistry at University College London. The availability of copious amounts of electricity at consistently low prices has attracted the global aluminium production industry to the country. The extraction of aluminium from ore is a very energy-hungry process. ‘Iceland imports aluminium ore, smelts it and then exports the aluminium again,’ Eric says.

In your class
This article presents some familiar chemistry in the context of the development of new carbon capture technologies. Pupils will regularly encounter the global problem of carbon dioxide emissions and climate change through news and popular media. You can use this article to enhance your teaching of combustion of alkanes, greenhouse gases, conservation of mass, and the rock, carbon and limestone cycles.
Volcano power
Eric has been overseeing a novel initiative to reduce emissions at the country’s largest geothermal power plant. Hellisheidi produces electricity and hot water from the Hengill central volcano, with a capacity of 300 MW of electricity and 120 MW thermal.
The initiative, CarbFix, is a carbon capture and storage (CCS) project with a twist. Conventionally, carbon dioxide captured using CCS is stored underground in depleted oil and gas reservoirs or other locations where it is unlikely to leak back out again. However, Iceland’s volcanic nature means it doesn’t have any nicely sealed underground reservoirs suitable for long-term gas storage. Eric’s team therefore needed to develop an alternative approach.
Instead, the team is injecting carbon dioxide captured at Hellisheidi into basalt, a reactive rock rich in divalent cations such as calcium and iron. Here it reacts to form the carbonate mineral calcite (CaCO3). This locks the gas away for millions of years in an environmentally benign manor. ‘The only solution for Iceland was to get the carbon dioxide to react with basalts to make carbonated rocks,’ Eric explains. ‘Once the carbon dioxide is mineralised it stays there forever: the average age of a carbonate rock in the crust is 200 million years old.’

The project started in 2006, and has been through many design and testing stages. In 2016, the team reported in Science surprising findings from its final pilot plant study at Hellisheidi. The carbon dioxide they had injected into the basalt had reacted to form rock in less than a year; it had been predicted this process would take many years.
But while the technology development has proceeded near perfectly, it hasn’t been a smooth ride getting this far. ‘There have been some twists and turns in the story,’ Eric says. ‘Over the years it became clear that there is no financial model to make carbon capture and storage work. Many of these projects globally started shutting down. It costs money to do and if no government was going to force people to do it, nobody would.’
The Icelandic government, however, was happy to fund the removal of a second hazardous gas from Hellisheidi’s flue gases: hydrogen sulfide (H2S). Unlike carbon dioxide, hydrogen sulfide has an immediate impact on the local population. ‘It smells of rotten eggs,’ Eric explains. ‘Because of increasing energy production the levels of hydrogen sulfide were beginning to get too high in some parts of Iceland. What we did is expand the capture and storage of carbon dioxide to capture and store, simultaneously, hydrogen sulfide and carbon dioxide.’ Hydrogen sulfide, when injected into basalt rocks, rapidly forms pyrite (FeS2), also known as fool’s gold. The hydrogen sulfide part of this project is called SulFix.
Since 2014, the CarbFix-SulFix project has been operating on a commercial scale. ‘About two-thirds of the gases produced by the plant are currently injected and this will be upscaled to 100% within about a year,’ Eric says. Without the capture technology, Hellisheidi emits about 40,000 tonnes of carbon dioxide and 12,000 tonnes of hydrogen sulfide each year. To put this in context, this is about 5% of the emissions that would come from a similarly-sized coal-powered plant.

Directed activity related to text (DART)
(Ages 11–16)
Exam questions requiring a longer written response (6–8 marks) often present familiar chemistry in an unfamiliar context and require students to read text and extract key information. Much of the chemistry in this article is familiar to 14–16-year-old students, though the idea of carbon capture won’t have been directly studied.
DART activities require pupils to engage with the text rather than just reading it passively. DARTs can be as simple as a set of questions related to a piece of text or a comprehension exercise. For older pupils, a focused task similar to the 6–8-mark examination questions is useful.
With activities like this, differentiation is important. Task 3a looks for advantages and disadvantages and is suitable for pupils who are beginning to develop this reading skill. Task 3b requires a higher level of analysis where pupils categorise phrases and summarise them.
Tasks
1. Read the whole article and underline unfamiliar words.
2. Once you have read the whole article once, reread the section headed ‘The technology’.
3a. Using different coloured pens, highlight phrases in the article that state the advantages and disadvantages of the CarbFix method of carbon capture.
3b. The issues surrounding scientific developments can be categorised into the ‘SEE’ framework covering social, environmental and economic impacts. Use the information in the article to summarise the impact of carbon capture into these categories.
The technology
The first generation of the CarbFix technology involved separating the carbon dioxide from the flue gases, dissolving it in water and then injecting it into basalt rock. Water reacts with carbon dioxide to form carbonic acid (H2 CO3), which plays an important role in the mineralisation process. In the final pilot study reported in Science, the gas from the plant was also combined with extra carbon dioxide brought in from elsewhere, including some spiked with heavy carbon (carbon-13) to aid monitoring of the mineralisation process. (Samples are still routinely taken from wells to monitor the pH and geochemistry at the injection site.)
The request to capture and inject hydrogen sulfide from the flue gases as well allowed the process to be simplified. ‘What we do now is take the exhaust gas from the power plant and put it through something called a sparger, which is a fancy name for a shower,’ says Eric. ‘Raining water on the exhaust causes the carbon dioxide and hydrogen sulfide to dissolve. We then take this pressurised water and inject it directly into the ground. It’s very simple.’ The process has been shown to work with both fresh and seawater.
Energy is the only major cost associated with the project once the system has been installed. ‘Energy is used in the inner pressurisation of system used to dissolve the carbon dioxide and hydrogen sulfide in the gas,’ Eric explains. But the amount of energy required is far less than for conventional CCS units that inject the gas into disused oil wells and the like. It has been estimated the CarbFix-SulFix process will use up approximately 0.2% for the power produced at Hellisheidi. This compares well to the 3 to 10% typically reported for conventional CCS units at coal- and gas-fired power plants. This equals significant cost savings. ‘The cost of doing this is approximately $25 a tonne, compared to in the order of $60 to $120 a tonne with conventional technology,’ says Eric.
The success of this mineralisation process opens the doors for the CarbFix-SulFix setup to be replicated at other power plants. ‘Basalts are very abundant,’ says Eric, both on land and under the sea. ‘Pretty much all the ocean floors are basalts, which is an advantage because people don’t seem to want carbon dioxide injected underneath their homes,’ he adds. Ultimately, however, Eric believes politics will determine whether the technology is eventually used elsewhere.
Meanwhile, in January 2017, his team started to develop their technology to capture carbon dioxide directly from the air. ‘Less than half of the carbon dioxide that goes into the atmosphere comes from power plants. More than half comes from cars, jet planes, etc. We’re going to have to air capture eventually,’ he says. Again, this technology would be suitable for any site near basalts and with lots of water available. ‘We’re teaming up with an air capture company to do this on the coastline of Iceland.’

Updated: the burning candle demonstration
(Ages 14–16)
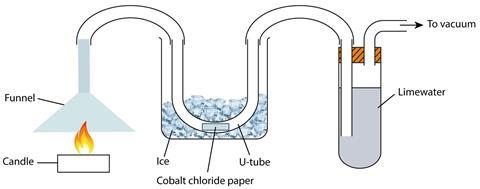
Carbon capture technologies are attempting to address the problem of carbon dioxide emissions. To appreciate the context of this article, students need to grasp the chemistry behind climate change and fossil fuels. This demonstration burns a candle in room air producing carbon dioxide and water, which are then captured by the apparatus.
Setting up the demonstration including two balances nicely illustrates the ideas around conservation of mass. A balance under the candle will show a mass decrease. A balance under the limewater will show a mass increase not equal to the mass lost from the candle. The mass changes can be measured during the demonstration at suitable intervals and graphs plotted.
You can then ask the students to consider why the mass changes are not the same. They should notice that the hydrogen from the waxy hydrocarbon has formed water in the combustion process and this isn’t being captured by the limewater. Students will tend to overlook that the mass of oxygen added to the carbon and the hydrogen atoms in the hydrocarbon is difficult to quantify, and also that other products (from incomplete combustion) may be formed and not trapped by the limewater.
This demonstration brings together lots of chemistry and can be used to revisit concepts from earlier topics. Questions that could be explored in discussion or written work include:
- What is the purpose of the ice around the u-tube?
- What does a colour change in the cobalt chloride indicate?
- What would happen to the mass of the candle and the limewater?
- How do the products of combustion change when the amount of oxygen available is varied?
- How many carbon atoms are in ‘wax’? Is wax a pure substance?
Download instructions for this demonstration from Learn Chemistry: rsc.li/2oUJXZh
Over the pond
Eric’s team are not the only ones looking at the potential of capturing carbon dioxide and turning it into rock. The CarbFix project is, however, by far the most advanced. To date, only one other team – the Big Sky Carbon Sequestration Partnership at Montana State University in the US – has taken the testing of this technology out of the lab and into the field.
In 2013, the Big Sky team, together with the not-for-profit organisation Battelle, injected around 1000 tonnes of carbon dioxide into basalt rock at a site in Washington State. ‘This was a moderate scale pilot where we injected purchased carbon dioxide, we didn’t capture it. It was to prove the principle,’ explains the project’s director Lee Spangler. ‘The purpose of the pilot was to field test injection of carbon dioxide into basalts, and see in situ what the chemical reactivity was, and how quickly that carbon dioxide would start turning into rock.’
‘The major difference between what we were doing and what has been done in Iceland is that the Icelandic team dissolve the carbon dioxide in water or brine before it is injected, whereas at the pilot we performed, it was injected as pure carbon dioxide gas,’ Lee says.
There is some brine or water in basalt rock naturally, but the absence of the step to pre-dissolve the carbon dioxide could change the chemistry of the mineralisation process. ‘Carbon dioxide dissolved in water becomes carbonic acid, and the reactivity of that can be different to injecting free phase carbon dioxide,’ says Lee. ‘Battelle laboratory tests showed it would still rapidly convert to minerals, but we wanted to check it in situ. The advantage of injecting it as pure carbon dioxide is that you don’t have to go through that step of dissolving it in water.’ Removing the pressurised water step will obviously reduce costs.
The pilot was a success, and sampling and monitoring at the site continued for a further 18 months following the injection process. The funding for this project has now ended and Lee’s team have turned their attention to conventional CCS. The so-called Kevin Dome Project is testing the potential of storing gaseous carbon dioxide long-term at an underground site in Montana.

Class practical: porosity of rocks
(Ages 11–14)
The porosity of rocks experiment is often used in studying the rock cycle – this article provides some additional context to that chemistry. In the classic experiment, rocks are soaked in bowls of water to determine the porosity. Using this article, the experiment can be given a new purpose as only porous rocks will be suitable for carbon capture. The aim of this experiment is to rank the rocks in order of their suitability and see if any of the samples in your school rock box is better than the basalt discussed here.
The experiment opens up some nice opportunities to introduce calculations and get pupils to think about relative measurements. Younger pupils will often initially think that the highest mass absorbed means the most porous rock and fail to take the size of the sample into account. The concept of relative measurement is important as pupils progress in chemistry and this is a good place to start looking at it.
Practical details
Prior to the experiment it is useful to test the various rocks in your sample box so you can make sure you get a good range of results. Pupils weigh the rocks and then soak them in bowls of water for 10 minutes. This downtime is a good point to read the article together (either in full or cut down into a couple of shorter paragraphs) in preparation for the analysis of results. You can give a results table, or pupils can design their own.
Analysis and conclusion
Once all the mass measurements have been taken, the pupils then need to carry out the calculations and determine the ranking. In writing the conclusion they need to revisit the original question – which rock is best for carbon capture? It’s also worth getting them into the habit of justifying their conclusions by linking to the data and, ideally, directly quoting it. For example, ‘Sandstone is the best rock for carbon capture as it is the most porous. This is shown by our experiment where it absorbed XX g of water per gram of rock, which was higher than the other samples.’
Higher-achieving pupils can be challenged to think about the assumptions made when coming to this conclusion. In this case, we assume the divalent cations needed for the reaction are present in all the rocks.
Download a student handout (pdf or MS Word), and teacher and technician notes (pdf or MS Word) for this experiment
Download a student handout, and teacher and technician notes for this experiment at rsc.li/EiC317-carbon-capture
How Mars turned to rock
Although the concept of capturing carbon dioxide from flue gases, or the air, and turning it into rock may sound a little out-there, it is a process that occurs naturally on Earth – albeit very slowly. Researchers in Glasgow also believe the same process may have been responsible for the dramatic thinning of the atmosphere on Mars a few billion years ago. The thin layer of gas that surrounds this planet contains around 95% carbon dioxide. Evidence, such as dried up river beds on its surface, suggests around 4 billion years ago the atmosphere was much thicker – thick enough to support life in fact.

It is not known for sure what happened to all that gas, but one theory is some of the carbon dioxide was sucked into rocks and mineralised. In 2013, scientists at the University of Glasgow reported evidence to support this claim; they found veins of carbonate minerals in a Martian meteorite thought to have landed on Earth around 3000 years ago.
‘In the laboratory we are now using high-pressure, high-temperature experiments to recreate approximately the Martian environment at that time,’ explains PhD student Adrienne Macartney. ‘The idea is to try to chemically and visually replicate the kind of carbonates we see in the Martian meteorites, with the general notion being if you’re chemically and physically replicating them, then perhaps the conditions you’ve used are similar to those on very early Mars.’
The similarities between these two research fields is obvious. ‘Mars is an example where we know a very similar process to CarbFix’s has happened naturally. But it’s happened on a very large, planetary scale. The areas we are looking at are hundreds of thousands of kilometres of potential reaction site, instead of just like couple of square kilometres in Iceland,’ Adrienne says.
Studying the natural process on Mars might also help us predict the potential impacts of CCS. ‘The work to understand the extent to which it potentially has affected the atmosphere on Mars might be helpful to help quantify the effects which this kind of technique might have on Earth. At the moment, there’s really not much dialogue between carbonate researchers on Mars and Earth.’
This is something Adrienne hopes to address. ‘We have an enormous amount of expertise in a lot of the same problems, so we may just find there’s a lot more efficiency in solving the terrestrial climate issues if we worked together.’

Debate: what if crude oil wasn’t running out?
(Ages 14–16)
This article raises an interesting philosophical debate: if we can capture carbon dioxide, does that mean we can continue to use and abuse our planet’s resources?
The ‘what if’ question stem is very useful for creating open questions that challenge students to really think about the issues presented. Pupils can often be intimidated by very open questions. For pupils who find this too challenging and whose thinking stalls, it can be worth presenting them with a framework to consider. The SEE framework (social, environmental, economic impact) is useful here.
This question can be presented as an essay title, a piece of group work or a class debate.
Download this article and all the teaching resources at rsc.li/EiC317-carbon-capture
Further reading
- The CarbFix project: bit.ly/carbfix-project
- J Matter et al, Science, 2016, 352, 1312 (DOI: 10.1126/science.aad8132)
- Basalt pilot project: bit.ly/big-sky-basalt
- University of Glasgow, planetary science and astrobiology research group: solarsystemrocks.org/current-research
- T Tomkinson et al, Nat. Commun., 2013, 4, 2662 (DOI: 10.1038/ncomms3662)
Downloads available for this article
- This article, without the classroom and teacher notes, as a pdf or MS Word file
- All the teaching ideas as a pdf or MS Word file
- Student handout for the DART exercise as a pdf or MS Word file
- Teacher/technician notes for the burning candle demonstration as a pdf
- Student handout for the rock porosity experiment as a pdf or MS Word file
- Teacher/technician notes for the rock porosity experiment as a pdf or MS word file
Article by Nina Notman, a science writer based near Baltimore, US. Teaching resources by Kristy Turner, a school teacher fellow at University of Manchester and Bolton School, UK
Will you use this article and resources with your students? What would make it more useful to you in the classroom? Let us know in the comments below, or send us an email: eic@rsc.org. |
Downloads
Full article
PDF, Size 61.17 kbFull article
Word, Size 58.16 kbTeaching ideas
PDF, Size 0.14 mbTeaching ideas
Word, Size 0.14 mbDART student handout
PDF, Size 40.8 kbDART student handout
Word, Size 53.47 kbRock porosity experiment student handout
PDF, Size 48.5 kbRock porosity experiment student handout
Word, Size 55.45 kbRock porosity experiment teacher and technician notes
PDF, Size 45.64 kbRock porosity experiment teacher and technician notes
Word, Size 54.58 kb









4 readers' comments