Dorothy Warren suggests ideas, activities and resources for your classroom

From mobile phones to the clothes we wear, everything consists of one or more of the 118 known chemical elements. So how do scientists know where to start when they are developing new or existing materials?
Chemists will often begin by making observations, then looking for patterns. They will use the periodic table to help as it is central to our understanding and classification of the world around us. It orders and classifies all the elements according to their physical and chemical properties.
What students need to know
All known elements are listed in the periodic table. The table features:
- rows called periods.
- columns called groups.
- metals on the left.
- non-metals on the right.
- reactive metals called alkali metals in group 1.
- the central metals block called transition metals.
- non-metals called halogens in group 7.
- unreactive gases called noble gases in group 0.
The elements in a group show patterns in their physical properties such as melting or boiling point, thermal conductivity and density. They have similar chemical reactions with, for example, oxygen.
The position of an element in the periodic table helps chemists to decide whether it will be useful for a particular job.
Download this
Download a collection of ideas and activities as MS Powerpoint or pdf.
In your class
Download a collection of ideas and activities from the Education in Chemistry website: rsc.li/2Z81sap
Ideas for your classroom
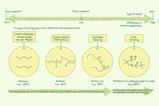
Analogies are a powerful way to help students understand the complexities of chemistry. We can use different classification systems from everyday life to help students understand how the periodic table classifies elements.
Start with analogies that students are likely to be familiar with, before moving on to less familiar examples. The layout of supermarkets is a good place to start. In a supermarket, food is grouped in different aisles according the characteristics and properties of the food – for example: fruit and vegetables, bakery, meat, dairy, cereals.
So, if you wanted to buy some butter, you would need to know this is a dairy product and go to the dairy aisle.
Introduce students to a range of different elements in the table by asking them to classify them as metals or non-metals. Then ask them to find the symbol on the periodic table. After repeating this with a number of elements, they will soon start to build up a picture and discover that metals are on the left-hand side of the table and the non-metals on the right.
Provide students with a blank periodic table. As keywords, ideas and elements are met each lesson, students add them into their table. Over time, this will help them build up their own picture of the periodic table, which emphasises the key points.

Next move onto contexts that show a link between a physical attribute and a property. A good example is the string section of an orchestra, which has different-sized instruments. The smallest instruments sit at one side and play the highest notes, while the biggest are at the other side and play the lowest notes.
Now take a look at a group of metals or non-metals such as the alkali metals or halogens. Demonstrate how the properties change as you go down the group. This can be done by looking up data such as melting points, and density and/or with practical demonstrations. Encourage students to look for patterns in reactivity and compare this reactivity to the position of the element in the periodic table.
You can also use the periodic table to develop students’ research and presentation skills. Assign each student a different element and ask them to find out and present key pieces of information to the group. Together you can build a class periodic table with this work. The RSC interactive periodic table is a good place to start researching.
There are lots of other resources available for consolidation and revision, such as Periodic table bingo, Elemental Su Duko, Element top trumps and the Periodic table game.
Common misconceptions
A common misconception is that all the elements are arranged on the modern periodic table by increasing atomic weight. While this was the starting point for many developers of periodic tables, closer inspection shows notable exceptions . The modern periodic table has elements arranged in order of atomic number, which is the number of protons in an atom whereas the atomic mass is based on the entire mass of the atom. To help overcome this alternative theory, encourage your students to look closely at the table and discover for themselves how the numbers increase. Emphasise that they should use the key to distinguish the relative atomic mass from the atomic number.
The position of hydrogen can often lead to confusion as different versions of the periodic table place it differently: at the top of group 1 or 7 or somewhere in between. The point to emphasis here is that hydrogen is an anomaly. It is a non-metal with an electronic structure similar to group 1 metals. Therefore chemists sometimes struggle to know where to place it.
A commonly used classroom activity is to identify and colour code elements which are liquids and gases at room temperature. While this helps students to familiarise themselves with the position of certain elements, it can lead to the misunderstanding that other elements can’t exist as gases and liquids. To challenge this idea, trying asking students to identify an alkali metal that is in the liquid state at 100°C.
An understanding of the concept of the particle nature of elements is essential to understanding how the periodic table works. Students can struggle with this, and it’s discussed in How to teach states of matter and particle theory.

Formative assessment
Use quick fire questions to find out what your students know. For example, name the element with the symbol He, or What is the symbol for potassium? Answers could be written on mini-whiteboards.
‘Odd one out’ activities require students to justify their choices, providing you within an insight into their thinking. For example, ask them to look at the images above and decide which one doesn’t fit.
Progression to 14–16
As students progress in their studies, they will be expected to describe the steps in the development of the periodic table. They will need to be able to explain how the position of an element in the periodic table is related to the arrangement of electrons in its atoms and hence to its atomic number. As well as, they should be able to explain the differences between metals and non-metals on the basis of their characteristic physical and chemical properties. From the position of elements in the table, they will need to be able to predict possible reactions and probable reactivity. They will be expected to explain how and why the reactivity of a group of element changes as you go down the group.
Take home points
- Students need to become familiar with the layout and keywords associated with the periodic table but don’t need to memorise it.
- Using a range of quick starter activities and games will support students in their learning of symbols.
- Ensure that students are secure in their understanding of basic chemical concepts, such as the particle model, physical and chemical properties, before asking them to identify trends within groups of elements.
- Students’ understanding of observed trends in the periodic table will develop as they learn and apply ideas about atomic structure.
Downloads
Introducing the periodic table
PowerPoint, Size 2.4 mbIntroducing the periodic table
PDF, Size 0.46 mb











5 readers' comments